Learn the definition of a replacement reaction or displacement reaction. Learn with examples of simple replacement reactions and learn how to use the reactivity series of metals to predict whether a reaction and the products will occur.
Definition of simple replacement reaction
A replacement reaction simple is a chemical reaction in which one element replaces another in a compound. It is also known as simple displacement reaction. The general form of a simple replacement reaction chemical equation is:
A + BC → B + AC
Simple replacement reactions occur when A is more reactive than B or the product AC is more stable than BC. A and B can be two metals (including the hydrogen(C is an anion) or two halogens (C is a cation). If BC and AC are in aqueous solutions, C acts as a spectator ion.
Examples of simple replacement reactions
There are two different scenarios for simple replacement reactions. In one form of reaction, one cation replaces the other. In the other form of reaction, one anion replaces the other.
Examples of cation replacement
Usually, the cation is a metal, but it does not have to be. Here are examples of simple replacement reactions involving cations:
- Zn (s) + 2 HCl (ac) → ZnCl 2 (ac) + H 2 (g)
- 2 K + 2H 2 O → 2 KOH + H 2 (note how the anion is written differently because we don’t write water as HOH)
- Cu + 2 AgNO 3 → 2 Ag + Cu (NO 3 ) 2
- Ca + 2 H 2 O → Ca (OH) 2 + H 2
However, if the reactant in element form is not more reactive than the other cation, no reaction takes place. In some cases, the reverse reaction is favoured, but not the direct reaction.
Examples of anion replacement
Instead of cation replacement, a single replacement reaction can involve the anion. In practice, the only anions involved in single replacement reactions are halogens (fluorine, chlorine, bromine, iodine). The general form of the reaction is:
A + BC → BA + C
In addition to being a simple replacement reaction, it is also an oxidation-reduction or redox reaction. Examples of anion replacement reactions include:
- Cl 2 + 2 NaBr → 2 NaCl + Br 2
- Br 2 + 2 KI → 2 KBr + I 2
Again, if the elemental reactant is not more reactive than the other anion, no reaction will occur. For example, the following reaction does not occur:
I 2 + 2 KBr → no reaction
How to determine the products of simple replacement reactions
The product of a single replacement reaction is fairly easy to predict. If the pure element is a halogen, it takes the place of the other halogen in the compound. All halogens have the same oxidation state (-1), so it is a simple substitution.
But, if the elemental reactant is not a halogen, it replaces the cation in the compound. The two cations do not always have the same oxidation state. You may have to balance the charge of the cation and the anion and then balance the chemical equation to get what you need.
For example, consider the reaction:
Mg (s) + AlPO 4 (aq) →
The magnesium is more reactive than aluminiumso the replacement is favourable. However, the aluminium cation has a charge of +3 (balancing the anion PO 4 3- ), while the magnesium ion (as a rare earth metal) has a charge of +2.
First, find the product formula by balancing the charges of cations and anions to obtain:
Mg (s) + AlPO 4 (aq) → Al (s) + Mg 3 (PO 4 ) 2 (aq)
Then, adjust the coefficients against the reactants and products to balance the chemical equation:
3 Mg (s) + 2 AlPO 4 (aq) → 2 Al (s) + Mg 3 (PO 4 ) 2 (aq)
Use of the reactivity series to predict whether a reaction will occur.
Use the reactivity series to determine whether a single replacement reaction will occur.
For anion replacement, the reactivity series for the halogens is:
Most reactive F 2 > Cl 2 > Br 2 > I 2 Less reactive
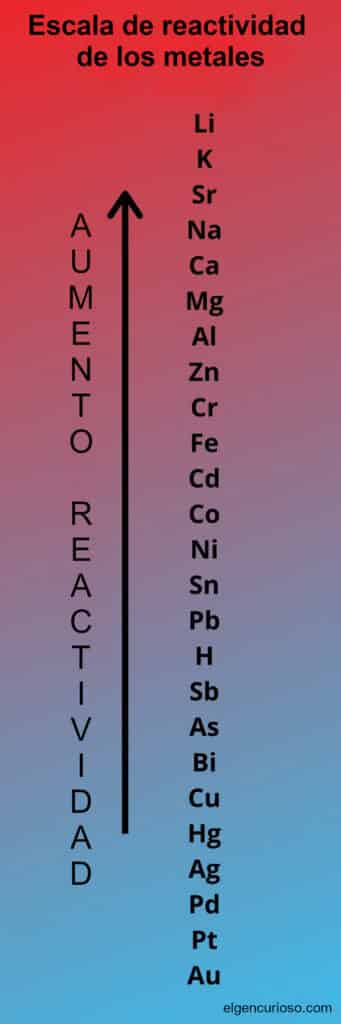
The elements lower on the list do not replace those higher on the list, so no reaction takes place.
This is the order of the halogens descending by their group in the periodic table, so it is easy to remember. The higher the halogen is on the periodic table, the more reactive it is. So, Cl 2 replaces I 2 in a single replacement reaction, but it will not react if the anion has fluoride ions.
The reactivity series of the cations is longer and not so obvious. The less reactive metals will not react with H + (aq), while the more reactive metals not only react with the ion, but can even extract the hydrogen ion from liquid water. Intermediate elements can react with the H + (aq) and sometimes extract hydrogen from water vapour.
But, for a general chemistry class, you mainly need to know which metals can replace each other and which cannot. For example, the zinc (Zn) can replace the tin (Sn) as a cation in a compound, but it cannot replace the potassium (K). In general, the alkali metals are the most reactive, followed by the alkaline earth metals. The noble metals, on the other hand, are relatively unreactive.
Learn more in the chemistry corner.