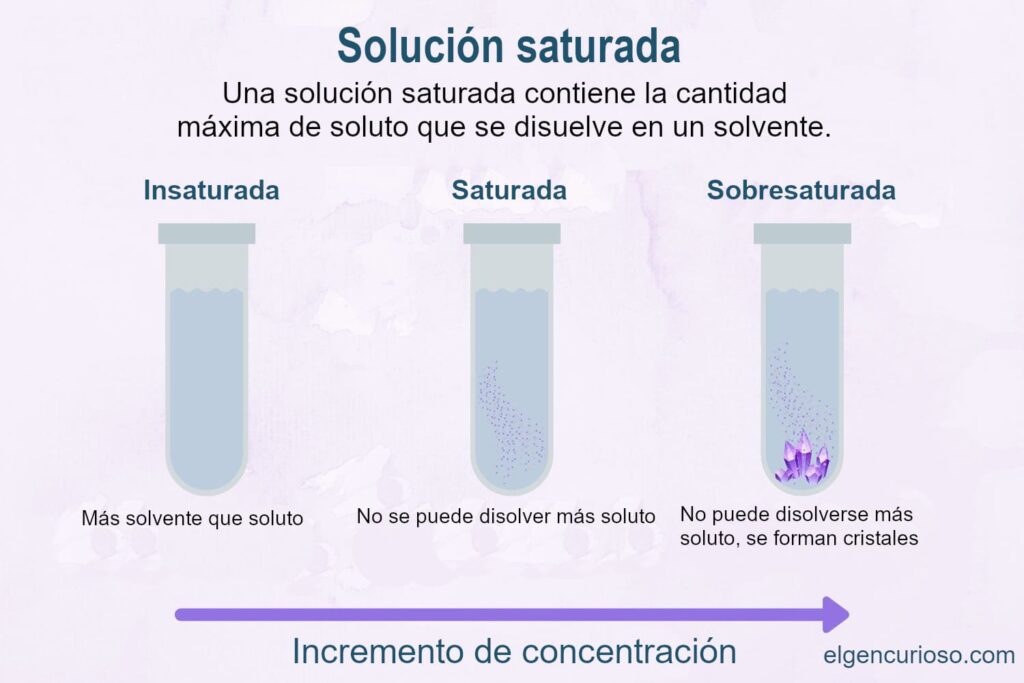
In chemistry, a saturated solution is a chemical solution that contains the maximum amount of solute dissolved in the solvent. The saturation point is the point of maximum concentration. Additional solute will not dissolve in a saturated solution and will not pass the saturation point.
Factors affecting saturation
The amount of solute that dissolves in a solvent depends on multiple factors. Some of the key factors affecting solubility are:
- Temperature: increasing temperature increases solubility, up to a certain point. For example, more salt dissolves in hot water than in cold water. A solution saturated at a cold temperature has a lower concentration than a solution saturated at a higher temperature.
- Pressure: the increase in pressure forces more solute into solution. One application is dissolving gases in liquids, such as carbon dioxide. carbon dioxide in soda.
- Chemical compositionThe nature of the solute and the solvent affect solubility. So does the presence of other compounds in the solution. For example, you can dissolve more sugar in water than salt in water.
- pHThe acidity or basicity of a solution affects whether ions dissociate or not, and thus influences solubility.
Saturated vs. supersaturated solutions
Controlling these factors allows for supersaturation. A oversaturated solution is an unstable solution that contains more solute than should be dissolved in the solvent. For example, if you prepare a saturated sugar solution in hot water and then cool the solution, it becomes supersaturated when the temperature changes. Disturbing the solution or adding a nucleation point (such as a seed crystal or even a scratch on the container) induces crystal growth.
Examples of saturated solutions
Saturated solutions are common in every life, not just in a laboratory! Here are some familiar examples:
- A soft drink is a saturated solution of carbon dioxide in water. When the pressure decreases as the container is opened, the solubility of the carbon dioxide decreases and it bubbles out of solution.
- Adding sugar to coffee or tea until it stops dissolving forms a saturated solution.
- Adding salt to melted butter until the grains stop dissolving forms a saturated solution.
- Honey is a saturated solution of sugars (glucose and fructose) in water. If you refrigerate honey, it crystallises because lowering the temperature reduces the solubility of the sugar.
- By stirring the cocoa powder mixture in water or milk until it stops, it dissolves and forms a saturated solution.
- You can add soap powder to the water until it no longer dissolves, forming a saturated solution.
How to make a saturated solution
There is more than one way to prepare a saturated solution:
- Add solute to a solvent until it no longer dissolves.
- Evaporate the solvent from an unsaturated solution until it reaches the saturation point.
- Add a seed crystal to a supersaturated solution to induce saturation. crystallisation. Excess solute is deposited on the crystal, leaving a saturated solution.
- In some cases, lowering the temperature of an unsaturated solution reduces the solubility of the solute enough to form a saturated solution.
What a saturated solution will not produce
There are two situations in which a solute and a solvent cannot form a saturated solution.
- Immiscible chemicals do not form solutions, saturated or not. For example, you cannot make a solution of oil and water because they do not mix. Similarly, you cannot prepare a solution of salt and paper. Neither chemical dissolves in the other.
- Likewise, fully miscible solutions do not form saturated solutions because, by definition, they combine in all proportions. For example, ethanol and water mix freely. There is no saturation point.
Basically, to form an unsaturated, saturated and supersaturated solution, you need a solute that is at least partially soluble in the solvent.