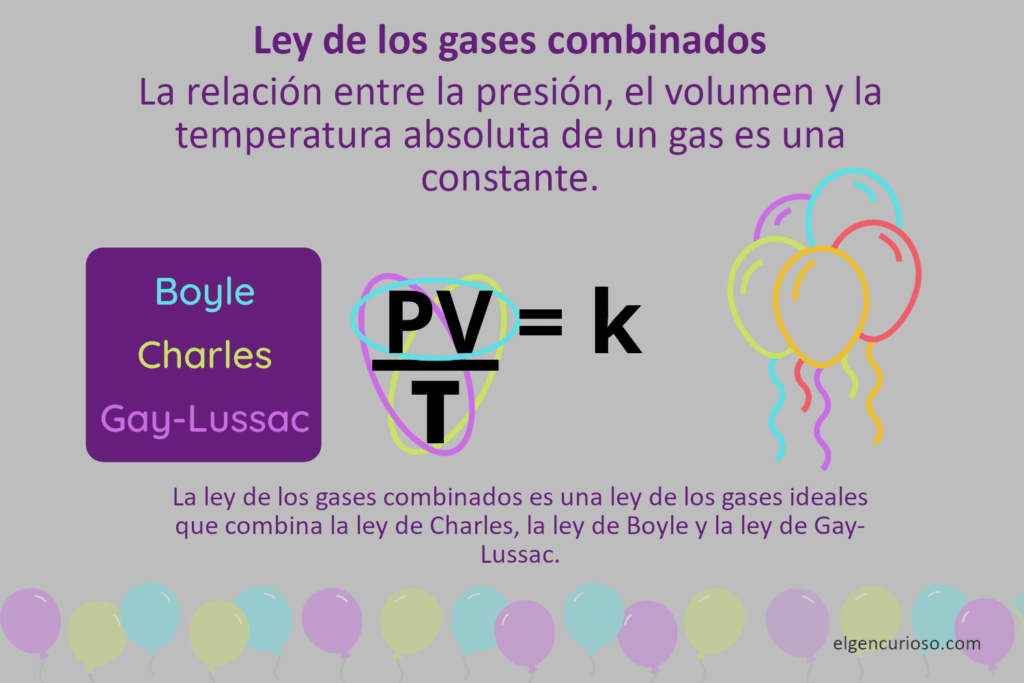
The combined gas law is an ideal gas law that combines Boyle’s law, Charles’ law, and Gay-Lussac’s law. It states that the ratio of the pressure-volume product to the absolute temperature of a gas is a constant. The pressure, volume and temperature are allowed to change, but the amount of gas (number of moles) remains unchanged. Basically, the combined gas law is the ideal gas law, except that it lacks Avogadro’s law. The combined gas law has no official discoverer, so it has no name.
- Combined gas law: PV / T = k
- Boyle’s law: PV = k
- Charles’ Law: V / T = k
- Gay-Lussac’s Law: P / T = k
Combined Gas Law Formula
The basic formula for the combined gas law related to pressure (P), volume (V) and absolute temperature (T):
PV / T = k
The constant k is a true constant, as long as the number of moles of a gas remains the same. If the amount of gas varies, k changes.
The practical formula of the combined gas law gives the “before and after” conditions of a gas:
P 1 V 1 / T 1 = P 2 V 2 / T 2
Rearrange the variables da:
P 1 V 1 T 2 = P 2 V 2 T 1
Any unit of pressure and volume is fine, but the temperature must be absolute. In other words, convert Fahrenheit and Celsius temperatures to Kelvin.
How the combined gas law applies to everyday life
The combined gas law has practical applications in everyday life. It applies whenever the amount of gas remains constant, but the pressure, volume and temperature change. For example, the law predicts the behaviour of cloud formation, refrigerators and air conditioners. It is also used in other thermodynamics and fluid mechanics calculations.
Because the combined gas law is an ideal gas law, it only approximates the behaviour of real gases. The law becomes less accurate at higher pressures and temperatures.
Example problem of the combined gas law
Find the volume of a gas at 760.0 mm Hg pressure and 273 K when 2.00 litres are collected at 745.0 mm Hg and 25.0 ° C.
First convert 25.0 ° C to the Kelvin scale. This gives 298 Kelvin.
Next, insert the values into the formula for the combined gas law. The most common mistake students make is to mix up the numbers that go together. Writing down what you are given helps to avoid this mistake:
P 1 = 745.0 mm Hg
V 1 = 2.00 L
T 1 = 298 K
P 2 = 760.0 mm Hg
V 2 = x (the unknown you are solving for)
T 2 = 273 K
Organise the formula to solve for the unknown:
P 1 V 1 / T 1 = P 2 V 2 / T 2
P 1 V 1 T 2 = P 2 V 2 T 1
V 2 = (P 1 V 1 T 2 ) / (P 2 T 1 )
Insert numbers:
V 2 = (745.0 mm Hg – 2.00 L – 273 K) / (760 mm Hg – 298 K)
V 2 = 1,796 L
V 2 = 1,80 L