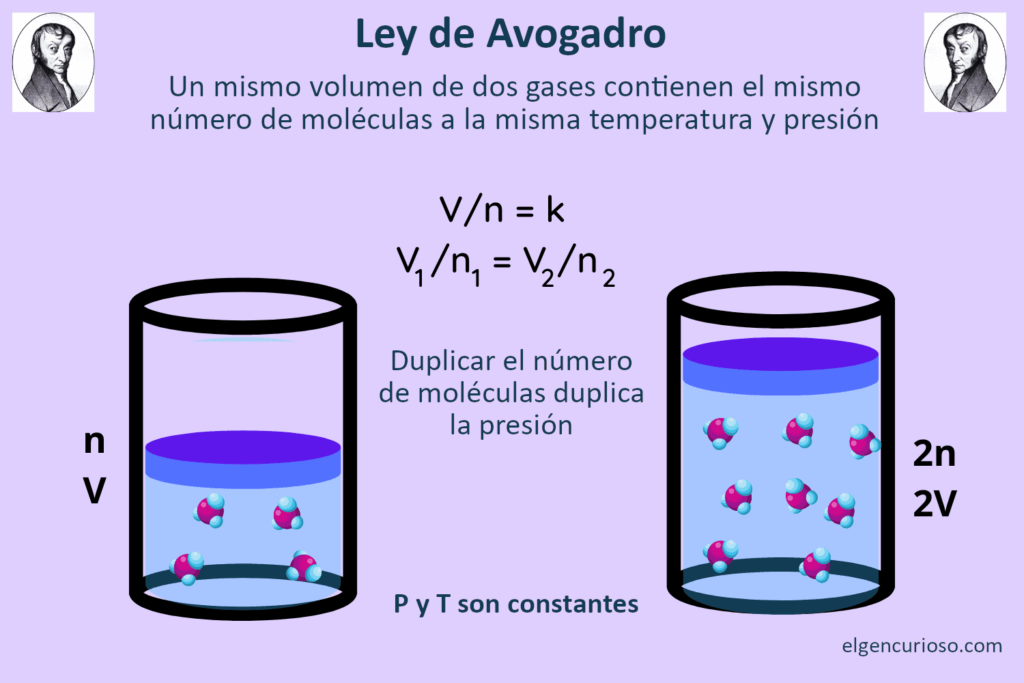
Avogadro’s law states that the volume of an ideal gas is directly proportional to the number of moles of gas, under conditions of constant temperature and pressure. As the number of moles of gas increases, the volume increases proportionally. This is independent of the size of the gas particles or their molar mass, so gases of different elements and compounds are comparable to each other.
Of course, as with any ideal gas law, the behaviour of real gases deviates slightly from the predicted behaviour. The law assumes that each gas particle has no volume and that the particles bounce off each other and their container under perfectly elastic conditions. Real gas molecules have volume and can be attracted or repelled by each other. Even so, Avogadro’s law is a useful approximation that is reasonably accurate for real gases under normal conditions.
History
The law is named after Amedeo Avogadro. In 1812, Avogadro hypothesised that two samples of ideal gas would contain the same number of molecules if they were at the same temperature and pressure. For example, a vial of gas hydrogen and a vial of gas nitrogen contain the same number of molecules at the same volume, temperature and pressure, although the gases have different identities.
Avogadro’s law is also known as Avogadro’s hypothesis or Avogadro’s principle. It is related to the other ideal gas laws: Boyle’s law (1662), Charles’ law (1787) and Gay-Lussac’s law (1808). The French physicist and mathematician André-Marie Ampère published the same law as Avogadro, but in 1814. In France, the ratio is called Ampère’s hypothesis, Avogadro-Ampère hypothesis o Ampère-Avogadro hypothesis.
Formula for Avogadro’s law
There are four common formulae that represent Avogadro’s law, where V is the volume, n is the number of moles of gas and k is a constant:
V∝ n
V / n = k
V 1 / n 1 = V 2 / n 2
V 1 n 2 = V 2 n 1
Because volume and number of moles are directly proportional to each other, a graph of volume versus number of moles is a straight line extending upwards from the origin.
Example of Avogadro’s law in everyday life
The best example of Avogadro’s law is inflating a balloon. The volume of the balloon increases as you add moles of gas. Similarly, when you deflate a balloon, the gas comes out of the balloon and its volume decreases.
Avogadro’s Law Example Problem
A 13.5 L volume of gas contains 0.000524 moles of nitrogen gas. Assuming the temperature and pressure of the gas remain unchanged, what volume will 0.00144 moles of the gas fill?
First, write down what you know and identify the unknown value:
V 1 = 13,5 L
V 2 =?
n 1 = 0.000524 mol
n 2 = 0.00144 mol
Next, insert the values into the formula for Avogadro’s law and rearrange the equation to calculate the answer:
V 1 / n 1 = V 2 / n 2
13.5 L / 0.000524 mol = V 2 / 0.00144 mol
V2 / 0,00144 mol = 13,5 L / 0,000524 mol
V2 = (13.5 L / 0.000524 mol) (0.00144 mol)
V2 = 37.1 litres