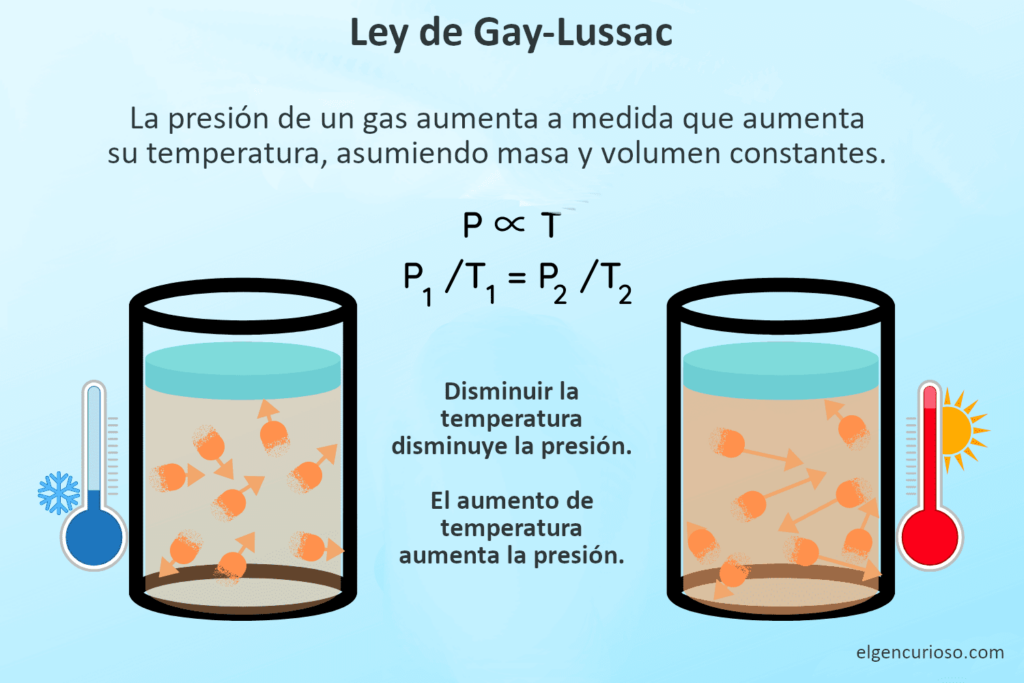
Gay-Lussac’s law o Amonton’s law states that the absolute temperature and pressure of an ideal gas are directly proportional, under conditions of constant mass and volume. In other words, heating a gas in a sealed container causes its pressure to increase, while cooling a gas reduces its pressure. The reason this happens is that the increase in temperature imparts thermal kinetic energy to the gas molecules. As the temperature increases, the molecules collide more frequently with the walls of the container. The increase in collisions is considered an increase in pressure.
The law is named after the French chemist and physicist Joseph Gay-Lussac. Gay-Lussac formulated the law in 1802, but it was a formal statement of the relationship between temperature and pressure described by the French physicist Guillaume Amonton in the late 17th century.
Gay-Lussac’s law states that the temperature and pressure of an ideal gas are directly proportional, assuming constant mass and volume.
Gay-Lussac’s Law Formula
Here are the three common formulas for Gay-Lussac’s law:
- P ∝ T
(P 1 / T 1 ) = (P 2 / T 2 )
P 1 T 2 = P 2 T 1
Where, P stands for pressure, while T is absolute temperature. Be sure to convert temperature in Fahrenheit and Celsius to Kelvin when solving Gay-Lussac’s law problems.
A graph of pressure versus temperature is a straight line extending upward and outward from the origin. The straight line indicates a directly proportional relationship.
Examples of Gay-Lussac’s law in everyday life
Here are examples of Gay-Lussac’s law in everyday life:
- Tyre pressureTyre pressure in car tyres drops on a cold day and rises on a hot day. If you put too much air in your tyres when they are cold, they may over-pressurise when they warm up. Similarly, if your tyres read the right pressure when they are hot, they will be underinflated when it is cold.
- Pressure cooker: applying heat to a pressure cooker increases the pressure inside the device. The increased pressure raises the boiling point of the water, shortening cooking times. Because the vessel is sealed, flavours are not lost in the air with the steam.
- Aerosol canThe reason why you should not store aerosol cans in hot conditions or dispose of them by burning them is because heating the can increases the pressure of the contents, which can cause it to explode.
- Water heater: An electric water heater looks very much like a pressure cooker. A pressure relief valve prevents steam build-up. If the valve does not work properly, the heat increases the pressure of the steam inside the heater and eventually causes it to explode.
Gay Lussac’s law problems
Example 1
A can of aerosol deodorant has a pressure of 3.00 atm at 25°C. What is the pressure inside the can at a temperature of 845 ° C? This example illustrates why you should not incinerate aerosol cans.
First, convert Celsius temperatures to the Kelvin scale.
T 1 = 25 ° C = 298 K
T 2 = 845 ° C = 1118 K
Then, insert the numbers into Gay-Lussac’s law and solve for P 2.
P 1 T 2 = P 2 T 1
(3.00 atm) (1118 K) = (P 2 ) (298 K)
P 2 = (3.00 atm) (1118 K) / (298 K)
P 2 = 11,3 atm
Example 2
Heating a gas cylinder to 250 K raises its pressure to 2.0 atm. What was its initial temperature, assuming the gas started at ambient pressure (1.0 atm)?
P 1 T 2 = P 2 T 1
(1.0 atm) (250 K) = (2.0 atm) (T 1 )
T 1 = (1.0 atm) (250 K) / (2.0 atm)
T1 = 125 K
Note that doubling the absolute temperature of a gas doubles its pressure. Similarly, halving the absolute temperature halves the pressure..
Other Gay-Lussac and Amonton’s laws
Gay-Lussac stated that all gases have the same average thermal expansivity at constant temperature and pressure. In other words, gases behave in a predictable way when heated. This law is sometimes also called Gay-Lussac’s law.
Usually, “Amonton’s law” refers to Amonton’s law of friction, which states that the lateral friction between two materials is directly proportional to the applied normal load, assuming a proportional constant (the coefficient of friction).