Intensive and extensive properties are the two kinds of physical properties of matter. A physical property, in turn, is one that can be observed and measured without changing the chemical composition of the sample. The physicist and physicist Richard C. Tolman coined the terms “intensive” and “extensive” in 1917. Here is an explanation of what intensive and extensive properties are, examples of each type and how to tell them apart.
Key points
Extensive and intensive properties are the two types of physical properties of matter.
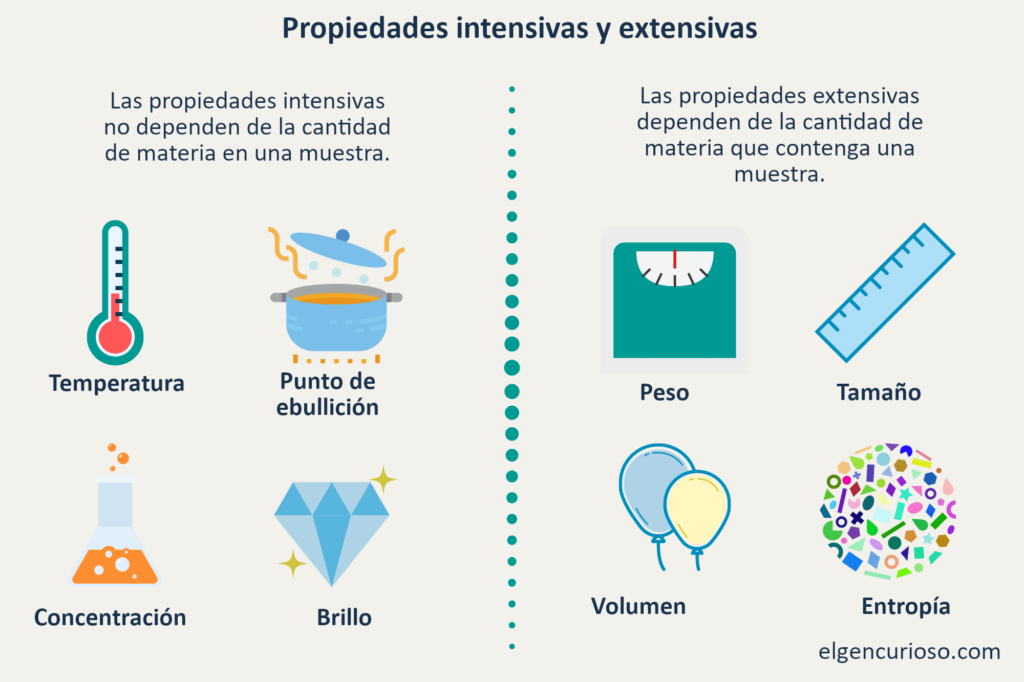
Intensive properties do not depend on the amount of matter in a substance. Examples include state of matter, temperature and density.
Extensive properties depend on the amount of matter in a sample. Examples include mass, length and volume.
Intensive properties
Intensive properties are also called bulk properties of intensive quantities. They do not depend on the amount of matter. These physical properties are used for sample identification because they are the same under different conditions and for all sample sizes. Examples of intensive properties include:
- Boiling point
- Colour
- Concentration
- Density
- Electrical conductivity
- Lustre
- Magnetic permeability
- Melting point
- Molality
- Smell
- Pressure
- Surface tension
- State of matter
- Temperature
- Refractive index
- Viscosity
Extensive properties
Extensive properties are physical properties that depend on the amount of matter in a sample. They are also known as extensive quantities. These properties are additive for subsystems. While extensive properties are not useful for sample identification, they are excellent for describing samples. Examples of extensive properties include:
- Energy
- Enthalpy
- Entropy
- Gibbs energy
- Heat capacity
- Length
- Mass
- Size
- Volume
- Weight
Specific properties
The relationship between two extensive properties is a special type of intensive property called a specific property. For example, mass and volume are both extensive properties. Their relationship is density, which is both an intensive property and a specific property. Other specific properties include specific volume (the reciprocal of density), specific heat capacity (heat capacity divided by mass), molar volume (volume per mole) and specific enthalpy.
How to differentiate between intensive and extensive properties
The easiest way to tell whether a physical property is intensive or extensive is to take two samples of the same type of matter and combine them. An intensive property will not change depending on the size of the sample. A small amount of matter has the same density, temperature and hardness as a large amount of the same substance. In contrast, an extensive property is additive. What this means is that doubling the sample size doubles a large property. So, doubling the sample would make it twice as massive, twice as long, etc.