States of matter are forms in which matter exists. The three states of matter observed in everyday life are solids, liquids and gases. Other states of matter also exist, although they require special conditions. Here is a look at the states of matter, their properties and the names of the phase transitions between them.
What is a state of matter?
Matter is anything that has mass and occupies space. It consists of subatomic particles, atoms, ions and compounds. Sometimes these particles are tightly bound and close together, while other times the particles are weakly connected and far apart. States of matter describe the qualities that matter exhibits. Basically, the state of matter of a substance depends on how much energy its particles have.
We can change the energy of matter by altering its temperature or pressure, causing matter to change from one state to another. But when matter changes state, its identity remains the same. So, if you take ice, melt it and then boil it, its state of matter changes, but it is always water.
List of states of matter
The four fundamental states of matter are solids, liquids, gases and plasma. But scientists are discovering new states of matter that exist under extreme conditions.
Solid
A solid is a state of matter with a defined shape and volume. The atoms, ions and molecules in a solid package are close together and can form crystals. Examples of solids include rocks, ice, diamonds and wood.
Liquid
A liquid is a state of matter with a defined volume, but without a defined shape. In other words, liquids take the shape of their container. The particles in a liquid have more energy than in a solid, so they are more separated and less organised (more random). Examples of liquids include water, juice and vegetable oil.
Gas
A gas is a state of matter that lacks a definite volume or a definite shape. Like a liquid, a gas takes the form of a container. Unlike a liquid, a gas expands or contracts easily to fill the entire volume of the container. The particles of a gas have more energy than those of solids or liquids. They tend to be farther apart and more random than in a liquid. Examples of gases include air, water vapour, and helium.
Plasma
Plasma is a state of matter similar to a gas, except that all particles are electrically charged. In addition, plasma tends to exist at very low pressure, so the particles are even further apart than in a gas. Plasma can consist of ions, electrons or protons. Examples of plasma include lightning, the aurora, the sun, and the interior of a neon.
Bose-Einstein condensate
The Bose-Einstein condensate (BEC) is sometimes called the fifth state of matter. In the Bose-Einstein condensate, atoms and ions cease to behave as separate particles and collapse into a single quantum state that can be described using a single wave function. This state of matter was verified experimentally in 1995 by Eric Cornell and Carl Wieman. The Bose-Einstein condensate is “colder” than an ordinary solid and can form very close to absolute zero.
Superfluid
A superfluid is a second liquid state formed by some types of matter. A superfluid has zero viscosity. Superfluidity was observed for helium in 1937. Because it could flow without friction, superfluid helium climbed up the walls of its container and dripped down the sides. Like the Bose-Einstein condensate, superfluidity occurs near absolute zero.
Fermionic condensate
A fermionic condensate is a state of matter similar to the Bose-Einstein condensate, except that it consists of fermions, such as quarks and leptons. Normally, the Pauli exclusion principle prohibits fermions from entering the same quantum state. In a fermionic condensate, a pair of fermions behaves like a boson, which allows several pairs to enter the same quantum state.
Rydberg matter
Rydberg matter is a type of plasma that forms when excited ions condense.
Photonic Matter
Photonic matter is the state of matter that forms when photons interact with a gas in such a way that the photons have an apparent mass and can interact with each other. Photons with apparent mass can even form photonic “molecules”.
Coloured glass condensate
Stained glass condensate is a state of matter that is proposed to exist when atomic nuclei travel close to liquid velocity. Because of its speed, the nucleus appears compressed along its direction of motion. This makes the gluons in the nucleus appear as an species wall or region of higher density.
Other states of matter
There are other proposed states of matter, which include quark matter, degenerate matter, dropleton, quantum Hall state, supervessel, supersolid, and string lattice liquid.
Phase transitions between states of matter
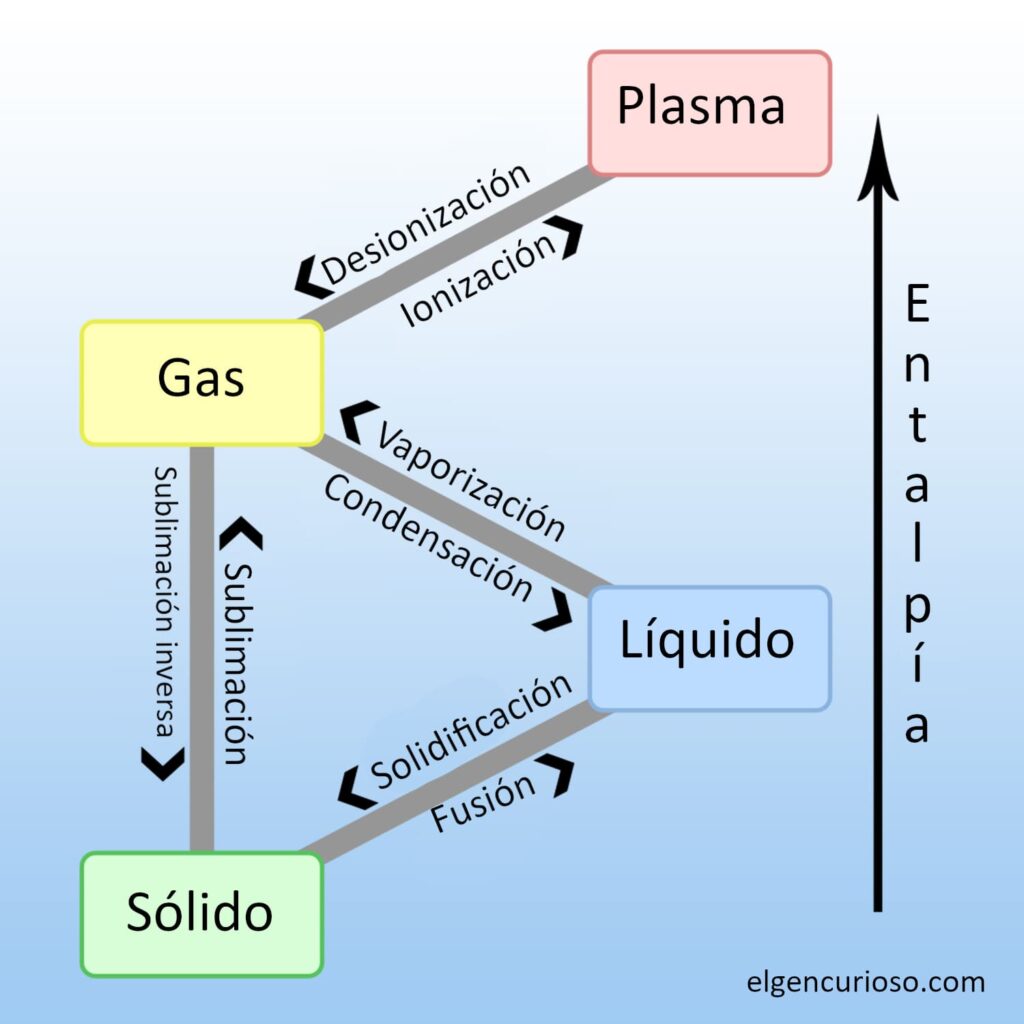
Changes in temperature and pressure cause matter to change from one state to another. This change is called a phase transition or phase change. Examples of phase transitions include the melting of ice (a solid) into water (a liquid) and the boiling of water into water vapour (a gas). These are the names for phase transitions between solids, liquids, gases and plasma:
- Melting: phase transition from solid to liquid.
- Freezing: phase transition from liquid to solid.
- Vaporisation: phase transition from liquid to gas.
- Condensation: phase transition from gas to liquid.
- Sublimation: phase transition from solid to gas.
- Deposition: phase transition from gas to solid.
- Ionisation: phase transition from gas to plasma.
- Deionisation or recombination: plasma-to-gas phase transition.