The molality is a measure of the concentration of a solute in a solution. It is mainly used when temperature is an issue. Molarity depends on the volume, but the volume can change when the temperature changes. The molarity is based on the mass of solvent used to create the solution because the mass does not change as the temperature changes.
This molarity example problem shows the steps necessary to calculate the molarity of a solution given the amount of solute and the mass of the solvent.
Problem
Calculate the molality of a solution prepared from 29.22 grams of NaCl in 2.00 kg of water.
Solution
The molarity is calculated by the formula:
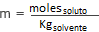
where moles SOLUTE is the number of moles of solute, in this case NaCl
and Kg Solvent is the mass in kilograms of the solvent.
First, calculate the number of moles of NaCl.
Using a periodic table, the atomic masses are:
Na = 22.99 g / mol
Cl = 35.45 g / mol
Add them together to get the molecular mass of NaCl.
molecular mass NaCl = 22.99 g / mol + 35.45 g / mol
molecular mass NaCl = 58.44 g / mol
Convert grams to moles of NaCl

moles of NaCl = 0.50 moles
Plug this and the mass of water into the molarity formula.

m = 0.25 moles / kg
o
m = 0.25 molal
Response
The molality of the NaCl solution is 0.25 molal.
As you can see, the molality calculations are straightforward. Remember to find the number of moles of solute and the mass of the solvent and the rest is simple. If you don’t know the mass of your solvent, you often know the volume. Use the density of the solvent to find the mass you need.