The molarity is a measure of the concentration of a solute in a solution. This molarity example problem shows the steps necessary to calculate the molarity of a solution given the amount of solute and the desired solution volume.
Problem
Calculate the molarity of a solution created by pouring in 7.62 grams of MgCl 2 in sufficient water to create 400 ml of solution.
Solution
The formula for calculating the molarity is:

In this case, the solute is 7.62 grams of MgCl 2. The formula needs the number of moles.
Using a periodic table, the molecular mass of MgCl 2 is 95.21 grams / mole. Find the number of moles in 7.62 grams in MgCl 2.
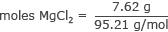
moles of MgCl 2 = 0.08 moles
The molarity must also have the volume in litres, not in millilitres.
The final volume of our solution is 400 mL, which is 0.4 L.
Insert this information into the formula
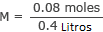
M = 0.2 moles/L or 0.2 M
Response
The concentration of MgCl 2 in solution is 0.2 M.